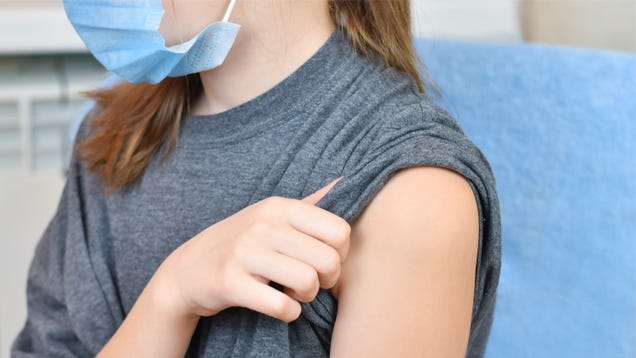
Pfizer and BioNTech announced today that they have completed a phase 3 trial of their COVID-19 vaccine in children aged 12 to 15, and that so far it seems to be 100% effective in that age group. If the FDA agrees that the vaccine is safe and effective, it might be available for ages 12 and up this summer.

0 commentaires:
Enregistrer un commentaire